| [1]Rey-Rico A,Silva M,Couceiro J,et al. Osteogenic efficiency of in situ gelling poloxamine systems with and without bone morphogenetic protein-2. Eur Cell Mater. 2011;21(4): 317-340.[2]Davis HE,Leach JK.Designing bioactive delivery systems for tissue regeneration.Ann Biomed Eng.2011;39(1):1-13.[3]Talwar R,Di Silvio L,Hughes FJ,et al.Effects of carrier release kinetics on bone morphogenetic protein-2-induced periodontal regeneration in vivo. J Clin Periodontol.2001; 28(4):340-347.[4]姚爱华,徐为,艾凡荣,等.中空羟基磷灰石微球作为rhBMP-2缓释载体的研究[J].无机材料学报,2011,26(9):974-978.[5]Fournier E,Passirani C,Montero-Menei CN,et al. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003; 24(19):3311-3331.[6]冯岚,郭健新,平其能,等.亮丙瑞林缓释微球的研究[J].中国新药与临床杂志, 2004,23(10):680-683.[7]张海龙,高玲美,邵洪伟.聚乳酸载药微球的制备及应用研究进展[J].西北药学杂志,2010,25(2):158-160.[8]林丽丽.聚乳酸载药系统的研究进展[J].广东化工,2012,39(6): 90-91.[9]Wen J,Kim GJ,Leong KW.Poly(D,L-lactide-co-ethyl ethylene phosphate)s as new drug carriers.J Control Release.2003; 92(1-2):39-48.[10]Chen L,Liu L,Li C,et al.A new growth factor controlled drug release system to promote healing of bone fractures: nanospheres of recombinant human bone morphogenetic-2 and polylactic acid.J Nanosci Nanotechnol.2011;11(4): 3107-3114.[11]费正奇,胡蕴玉,吴道澄,等. rhBMP-2/聚乳酸与聚乙醇酸共聚物载药微球的制备及成骨活性研究[J].生物医学工程与临床,2006, 10(3):121-124.[12]Reichert JC,Saifzadeh S,Wullschleger ME,et al.The challenge of establishing preclinical models for segmental bone defect research.Biomaterials. 2009; 30(12): 2149-2163.[13]Silva AM,Souza WM,Koivisto MB,et al.Miniplate fixation for the repair of segmental mandibular defects filled with autogenous bone in cats.Acta Cir Bras. 2011;26(3):174-180.[14]Langer R,Vacanti JP.Tissue engineering. Science.1993; 260 (5110):920-926.[15]Crane GM,Ishaug SL,Mikos AG.Bone tissue engineering. Nat Med.1995;1(12): 1322-1324.[16]刘昊,张永刚.骨组织工程的研究应用与进展[J].生物骨科材料与临床研究, 2012,9(2):32-34.[17]Urist MR.Bone: formation by autoinduction. Science.1965; 150(3698):893-899.[18]丁立峰,周振东,杨军,等.骨形态发生蛋白2基因转染的兔骨髓间充质干细胞复合聚乳酸-聚己内酯支架体外构建载基因仿生骨的实验研究[J].中国矫形外科杂志, 2013,21(9):918-923.[19]Zuk PA,Zhu M,Mizuno H,et al.Multilineage cells from human adipose tissue: implications for cell-based therapies.Tissue Eng.2001;7(2):211-228.[20]侯锐,毛天球,陈书军,等.rhBMP-2及rhbFGF对兔骨髓间充质干细胞分化作用长期效应的实验研究[J].医学研究生学报,2004, 17(8):694-697.[21]Schmidmaier G,Schwabe P,Strobel C,et al.Carrier systems and application of growth factors in orthopaedics.Injury. 2008; 39(Suppl 2):S37-S43.[22]Hong SJ,Yu HS,Kim HW.Tissue engineering polymeric microcarriers with macroporous morphology and bone-bioactive surface. Macromol Biosci. 2009;9(7): 639-645.[23]张洁琼,曹健,李艳芳,等.聚乳酸的合成•生物降解及应用研究现状[J].安徽农业科学,2010,38(31):17384-17386.[24]黄玲玲,姜志华.PLA的合成及改性研究进展[J].合成树脂及塑料, 2010,7(6): 65-69.[25]Mi FL,Shyu SS,Lin YM,et al.Chitin/PLGA blend microspheres as a biodegradable drug delivery system: a new delivery system for protein.Biomaterials.2003; 24(27):5023-5036.[26]曲昌锋,陆平,周晓南,等.自身增强聚乳酸接骨板在颌骨骨折中的临床应用体会[J]. 中国实用口腔科杂志,2009,2(11):690-691.[27]Ogawa Y,Yamamoto M,Okada H,et al.A new technique to efficiently entrap leuprolide acetate into microcapsules of polylactic acid or copoly(lactic/glycolic) acid.Chem Pharm Bull (Tokyo).1988;36(3):1095-1103.[28]中华人民共和国药典2010年二部[S].国家药典委员会,2009.[29]Suzuki K,Nakamura T,Matsuura H,et al.A new drug delivery system for local cancer chemotherapy using cisplatin and chitin.Anticancer Res.1995;15(2): 423-426.[30]Jule E,Nagasaki Y,Kataoka K.Lactose-installed poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles exhibit fast-rate binding and high affinity toward a protein bed simulating a cell surface. A surface plasmon resonance study.Bioconjug Chem.2003;14(1):177-186. |
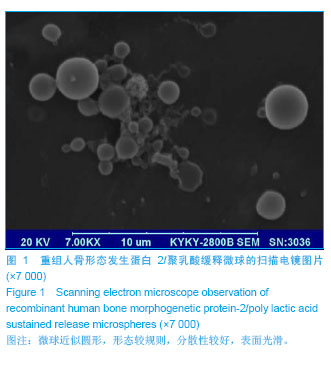
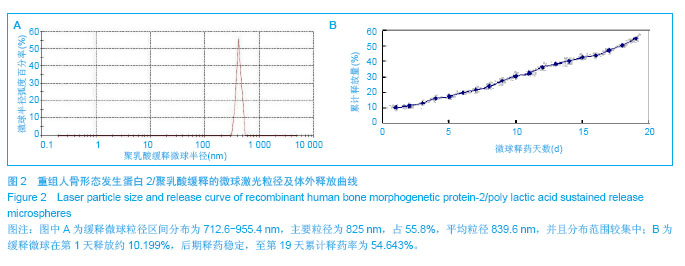
.jpg)